2019: INTERNATIONAL YEAR OF THE PERIODIC TABLE
by Sergio Cristallo
Obviously, the cosmic nucleosynthesis does not stop at the elements previously described. In fact, in massive enough stars (at least 10 times the solar mass), the central temperature is so large to trigger a large sequence
of α captures. As a consequence, heavier and heavier elements are synthesized:
neon (20Ne), magnesium (24Mg), silicon (28Si), sulfur (32S),
argon (36Ar), calcium (40Ca), titanium (44Ti), chromium (48Cr),
iron (52Fe) and, finally, nickel (56Ni). The latter is unstable and decays to its stable isobar 56Fe
in about 70 days (passing through 56Co).
As it is shown in Figure 6, iron has one of the lowest mass per nucleon (in other words, it is one of the most stable nuclei, because it has a very large binding energy per nucleon).
Basically, it means that the production of isotopes heavier than 56Fe through charged particles (as those previously described) needs energy, instead of releasing it (i.e. the process becomes "endoenergetic").
Moreover, the Coulomb repulsion increases with the charge of reactants (i.e. the number of protons of the two interacting nuclei). Therefore, elements heavier than iron cannot be produced via charged particle reactions.
Figure 6: THE STABILITY OF CHEMICAL ELEMENTS
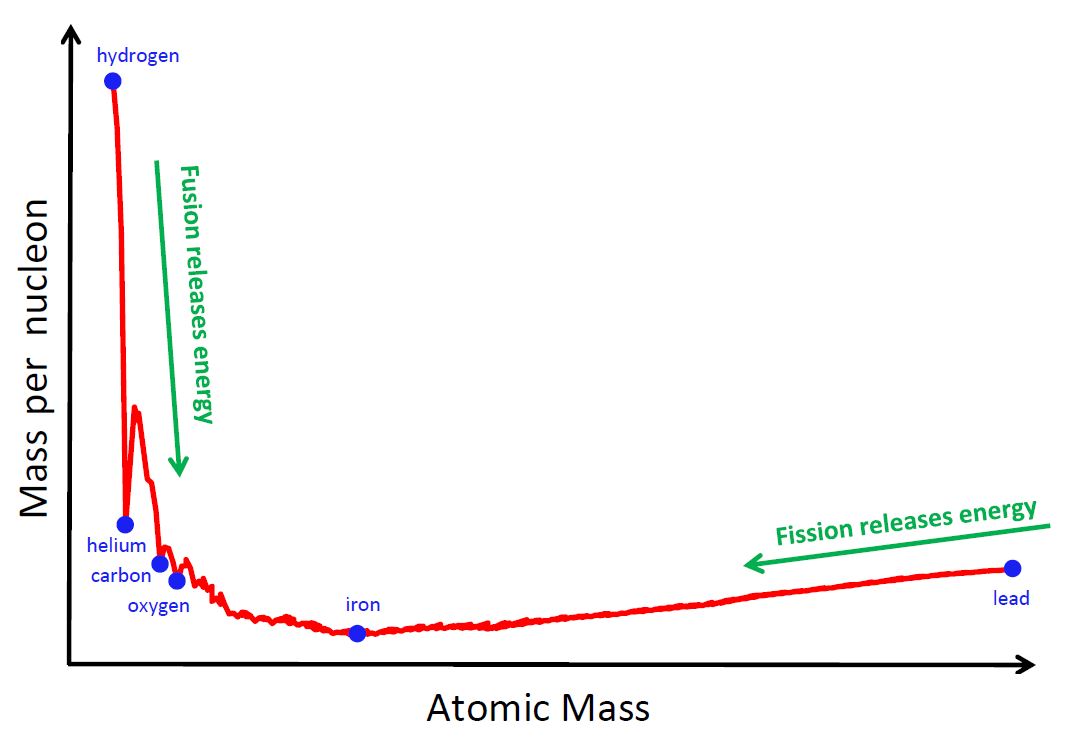
An alternative solution may come from nuclear fission.
While in a fusion process two nuclei merge in a single larger nucleus, during the fission the contrary occurs, i.e. an heavy nucleus breaks down in smaller components, releasing energy
(this process is the heart of nuclear reactors).
The problem is that the fission process requires the presence of very heavy elements (the so-called Actinides),
which are exactly the elements we would like to produce. Therefore, fission cannot solve our problem.
The theory described above is confirmed by the distribution of chemical elements on the solar surface (previously shown in Figure 3).
As it can be easily noted, iron presents a peak (it is the 5th most abundant element in the Universe, after hydrogen, helium, oxygen and carbon).
